Nexocyte Platform
Fluicell represents a new paradigm in therapeutics. Our Nexocyte™ tissue production platform pushes the boundary of what is possible within regenerative medicine and drug development, enabling completely new ways to treat disease.
Chronic disorders related to tissue damage, including cardiac, pulmonary, kidney and metabolic disorders, are among the diseases responsible for the most deaths around the world. With our tissue production platform, we are unlocking new ways to treat these diseases, offering ways to create therapies that can improve the lives of millions of patients worldwide.
Fluicell’s Nexocyte platform is based on twenty years of R&D and combines our high-resolution bioprinting technology with state-of-the-art biomaterials and tissue design tools and know-how. The unique technology makes it possible to control tissue formation down to the level of individual cells.
No matter whether the goal is to create tissue-based therapeutic applications or human screening products, our platform can deliver detailed tailored tissue-based solutions, targeting any disease area and using any cell type.
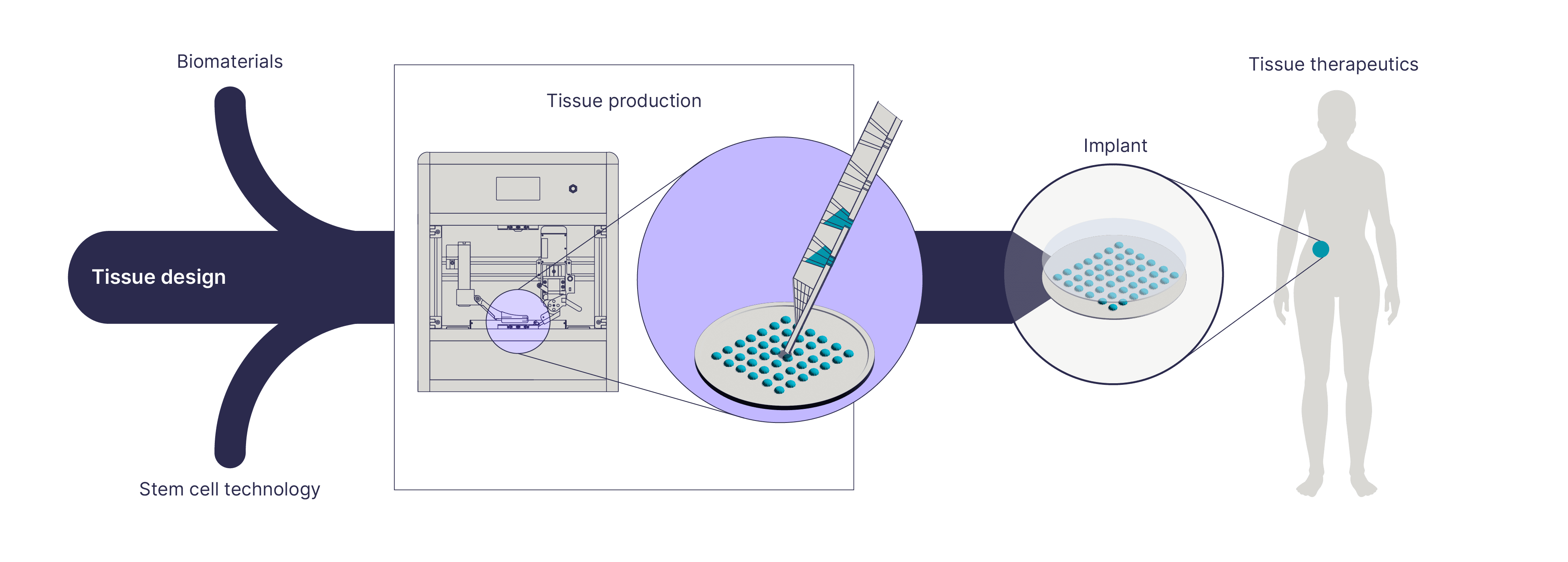
Unlock therapeutics with Nexocyte tailored tissues
The ability to create tissues using any cell type with single-cell precision opens a whole new world of possibilities for regenerative medicine and drug development. With our Nexocyte platform, the possibilities for design-driven tissue engineering are almost endless.
At the core of the platform is Fluicell’s bioprinting technology Biopixlar, which makes it possible to build functional tissues directly using the cells you want – without the need for any bioink or other carrier material. Thanks to its high-precision cell deposition technology, you can bring your detailed tissue designs to life without having to compromise. The high-resolution bioprinting capacity makes it possible to place cells close to each other, ensuring efficient cell-to-cell communication.
Watch: PRINTING WITH BIOPIXLAR
Engineered pancreatic islets containing alpha and beta cells, printed on biomaterial substrate using Biopixlar.

Biopixlar® single-cell bioprinting
Biopixlar is a completely new type of bioprinter with the unique capability to position cells in three dimensions with high resolution and precision without the use of bioink. Based on innovative Fluicell technology, Biopixlar is capable of generating detailed, multicellular biological tissues, directly in cell culture media.
Biopixlar lets you print even the most demanding cell types in any configuration, from single-cell arrays to organoids and complex tissues. The high-precision microfluidic cell deposition technology promotes intercellular communication and ensures high cell viability.
Biopixlar has been used to print hepatocytes, beta cells, cardiomyocytes, T-cells, primary neurons, IPSCs, MSCs, corneal cells, melanoma and adenocarcinoma cells, neuroblastomal cells, fibroblasts, renal epithelial cells, keratinocytes, and much more.

Single-cell tools
Fluicell has developed research tools based on our unique microfluidic technology that make it possible to study individual cells directly in their native environment and to perform biological assays with high precision.
Our single-cell toolbox contains the devices BioPen, Biozone 6 and Dynaflow Resolve. The devices add a wide range of capabilities to our Nexycyteplatform, including localized drug delivery, dose response assays andsingle-cell analysis. Fluicell's single-cell technologies work with any cell type and require very little material, making them ideal for scarce and valuable samples.
Intellectual property
Fluicell’s tissue engineering technology is covered by a broad IP platform, focused on controlling liquid flows, cells and biology with high precision. Our IP strategy is directed towards protecting the enabling methods and technologies that makes it possible for us to develop innovations in a wide range of therapeutic and application areas.
Read more
PartnershipS
Fluicell’s mission is to transform medicine with next-generation regenerative medicine and screening products. Partner with us to change how we treat diseases.
Pipeline
Our pipeline consists of products based on our tissue production platform, including tissue-based therapeutics for type 1 diabetes treatment, and cardiac toxicity and kidney disease screening models.


Partner with us!
Learn about what we hope to achieve in partnership with innovation-driven pharmaceutical companies. Have questions or interested in exploring a collaboration? Get in touch.